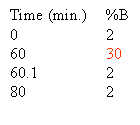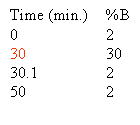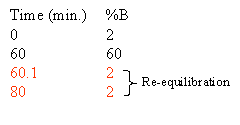Learn about mass spectrometry from top MS experts, sign up for a free one hour webinar
LC/MS를 위한 역상 HPLC 기초
Ion Source 지침서
저자: Andrew Guzzetta
This Tutorial was first published
July
22nd, 2001,
and was translated into Korean in 2010
by
Sun Y. Kim
IonSource
Homepage | Disclaimer
read
important laboratory safety notice at bottom of page before proceeding
원래는 이 지침서를 ‘프로테오믹스를 위한 역상 HPLC’로 부르려고 하였으나 좀 더 제한적인 경우를 연습해 보기로 하였다. 이 지침서를 쓰게 된 이유는 역상 크로마토그라피 (reverse phase chromatography)가 LC/MS 응용에 사용되는 가장 일반적인 크로마토그라피법이기 때문이다. 이 지침서는 학생과 역상 크로마토그라피, HPLC, LC/MS를 처음 사용하려는 사람을 대상으로 쓰여졌다. 여기서는 펩타이드와 단백질의 역상 크로마토그라피를 다루었지만 그 원리는 어느 혼합물의 크로마토그라피에도 응용될 수 있다.
목차 |
1. 서론 이 지침서가 전하는 메세지는 역상 크로마토그라피는 정말 간단하다는 사실이다. 혼합물은 고농도 수용성 이동상 (mobile phase)에서 역상 HPLC 컬럼에 흡착되고 고농도 유기성 이동상에서 역상 HPLC 컬럼로 부터 용출 된다. 역상 HLPC에서 혼합물은 소수성 (hydrophobic character)에 의해 분리된다. 펩타이드는 유기 용매의 선형 그레디언트 (linear gradient)에 의해 분리될 수 있다*. 모르는 물질을 분리할 때는 60/60 그레디언트로 분리할 것을 동료들에게 권한다. 60/60 그레디언트란 100% 수용성에서 출발해서 그레디언트가 60분만에 60% 유기 용매로 증가되는 것을 뜻한다. 대부분의 펩타이드 (길이 10-30 아미노산)는 30% 유기 용매에서 대부분 용출된다. 간단한 역상 HPLC 원리는 다음과 같다.
|
| The HPLC
사용하려는 HPLC는 대부분의 경우 두 용매를 펌프하고 혼합할 수 있어야 한다. 이 기능은 한개의 펌프와 상응하는 밸브나, 또는 두 개의 펌프에 의해 실행될 수 있다. 펌프 모양은 속이 투명한 기기 모양이 일반적이다. 역상 크로마토그라피는 용매 조건이 일정하게 유지되는 일정 용매 조성법 (isocratic mode)에서도 실행될 수 있는데** 이 경우 한개의 펌프에 의해 실행된다. 일정 용매 조성법은 단일 물질의 특징을 자세히 결정하거나, 혼합물의 조성이 무엇인지, 관련된 분해 부산물을 찾는 QC의 경우에 많이 사용된다. 주변에 HPLC가 없을 경우 HPLC 공급자 정보가 여기에 있다. Link to HPLC vendors and accessory suppliers.
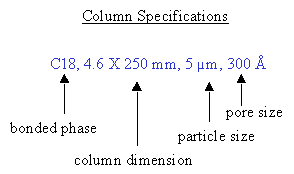
a. 컬럼 크기 (dimension) b. 입자 크기 및 기공 크기 (particle size and pore size) c. 정지상 (stationary phase)
a.
컬럼은
보통
내부
직경x길이
(4.6mm x 250mm)의
형태로
표시된다.
질량
분석법에서
쓰이는
컬럼
내부
직경은
보통
0.05-4.6mm이나
대용량을
준비할 때 더
크다.
질량
분석법에서는
짧은
컬럼이
긴
컬럼만큼
요긴하며
오히려
짧은
컬럼이
경제적이고
역압 (back
pressure)도
작다.
역압이
낮은
건
왜
중요할까?
압력이
낮을
경우
분석시
흐름
속도
(flow rate)를
신축성
있게
잘
조절할
수
있기
때문이다.
흔히
짧은
컬럼은
보통보다
높은
흐름
속도에서
빠른
크로마토그라피를
할
때
사용된다.
길이면에서
보통
100mm
컬럼을
사용하지만
50mm, 30mm
컬럼도
필요에
따라
LC/MS
분리에
사용되기도
한다.
b. 대부분
컬럼은
실리카
입자로
충진된다.
비드
(bead)나
입자들은
입자와
기공
크기에
의해
성질이
결정된다.
입자
크기는
보통
3-50 micron (µm)인데
5 micron
입자가
펩타이드
용으로
가장
많이
이용된다.
큰
입자는
시스템
압력을
낮게
만들고
작은
입자는
시스템
압력을
높게
만든다.
일반적으로
입자가
작을수록
분리
효율이
높다.
입자의
기공
크기
단위는
angstrom (A)이고
일반적인
범위는
100-1000 angstrom이다.
단백질
펩타이드용으로
300 angstrom이
가장
많이
쓰이고,
small molecule용으로는
100 angstrom이
가장
많이
쓰인다.
실리카는
가장
흔한
입자
재료이다.
실리카는
높은
pH에서
녹기
때문에
pH=7가
넘는
용매에는
쓰이지
않는다.
그러나
최근
높은
pH를
견디는
실리카
관련
기술이
소개되고
있으므로
여러
제조사들이
권유하는
것을
따를
필요가
있다.
특히
높은
온도와
강한
pH를
동시에
쓸
경우
실리카를
손상시킬
수
있다.
5 micron 실리카 입자는 지름이 50,000 angstrom이다. 이 입자의 적도를 따라 166,300 angstrom 기공을 채울 수 있다. c. 정지상(stationary phase)은 일반적으로 분석물과 결합하는 소수성 alkyl 체인 (-CH2-CH2-CH2-CH3)으로 이루어져 있다. 가장 많이 쓰이는 체인 길이는 C4, C8, C18이다. C4는 단백질에, C18는 펩타이드나 small molecule에 흡착에 사용된다. 그 원리는, 크기가 큰 단백질은 컬럼과 결합시에 더 소수성인 성질을 띠는 경향이 있으므로 짧은 alkyl 체인이 적당하다. 반면 펩타이드는 작기 때문에 결합시에 길이가 더 길고 소수성인 체인이 필요하고 따라서 C8, C18가 펩타이드나 small molecule에 적합하다. 흥미로운 사실은 C8 컬럼이 작고 수용성인 펩타이드를 더 잘 흡착한다는 사실인데, 그 원리는 C18 체인이 그레디언트의 초기 수용성에 lay down되므로 보다 수용성 펩타이드는 부착되지 않는다. 우리는 모든 펩타이드에 주로 C8을 쓰는데 C18과 적어도 동등하거나 C8보다 나은 선택이다 .
The reverse phase solvents are by convention installed on the HPLC channels A and B. The A solvent by convention is the aqueous solvent (water) and the B solvent by convention is the organic solvent (acetonitrile, methanol, propanol). It is important to follow this convention since the terms A and B are commonly used to refer to the aqueous and organic solvents respectively. The A solvent is generally HPLC grade water with 0.1% acid. The B solvent is generally an HPLC grade organic solvent such as acetonitrile or methanol with 0.1% acid. The acid is used to the improve the chromatographic peak shape and to provide a source of protons in reverse phase LC/MS. The acids most commonly used are formic acid, triflouroacetic acid, and acetic acid. A 0.1% v/v solution is made by adding 1ml of acid per liter of solvent. Triflouroacetic acid has been reported to suppress MS ionization and often mass spectroscopists lower the percentage of TFA to 0.05 or even 0.02% without significant loss in chromatographic efficiency. Some MS people add a small percentage of heptafluorobutyric acid (HFBA, pdf from Michrom) to acetic acid solvents or low TFA containing solvents to help improve peak shape. Since modern mass spectrometers are very sensitive it is important not to use plastic pipette tips when adding acid to the mobile phase, always use glass. In our work we use acetonitrile as our organic solvent. We have heard that the best electrospray solvent is 30% methanol, 35 mM acetic acid. We commonly use this solvent system for ESI MS infusion, but have found that acetic acid is an inferior acid for chromatographic peak shape. Our preferred HPLC grade water, acetonitrile and methanol is purchased from Burdick and Jackson. Our preferred TFA comes in 1 ml ampoules from from Pierce Chemical Company.
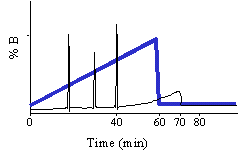
When chromatographing an unknown we normally use the following simple gradient to learn about the hydrophobic character of the unknown compound. The % A in the gradient described below is implied. We call this the 60/60 gradient, because we run from near 0% B to 60% B in 60 minutes. Through experience we have noted that 90% of all peptides will elute from a C18 reverse phase column by 30% acetonitrile. There may be a few really hydrophobic peptides that elute later that is why we take the gradient to 60% B. You may even want to run this gradient to 80% at least once to see if you are getting everything off of the column. You may ask why don't we start the gradient at 0% B? As we talked about before, in 0% organic and in high aqueous, the very hydrophobic, long C18 alkyl chains in an effort to get away from the high aqueous environment mat down on the particle. When these alkyl chains mat down they are inefficient at capturing the analyte so chromatographers in the know start the gradient with some small % of organic, 2-5%.
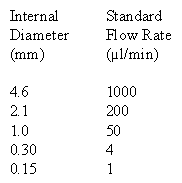
It is important to use the correct flow rate for your HPLC column. Below is a table with standard flow rates for easy reference. If you are running a column with a different diameter than those shown in the table please review the maintaining linear velocity page to learn how to calculate the appropriate flow rate for your column.
Based on the separation shown at the top of this section one could rewrite the gradient to look like this:
The column must be equilibrated, re-equilibrated to the initial high aqueous solvent composition before another analysis can be performed. Normally this re-equilibration is stuck onto the end of the gradient. How much equilibration time is enough? As a rule of thumb we give 20 minutes. In reality it depends on the column length, flow rate and the hydrophobicity of your peptides. Some chromatographers use 10 minutes as their standard equilibration time. Equilibration is all about fitness of purpose. You should determine the the equilibration time experimentally, the criteria will be, does my analyte really stick to the column and chromatograph appropriately and reproducibly with subsequent analyses. If you choose to do this part of the method development you will undoubtedly be rewarded with improved chromatography and better cycle time.
Should I Control Column Temperature? Yes. Scientists are control freaks. If you can control a variable, control it! Actually if you are performing automated analyses over a long period of time peak retention times can drift with changing ambient temperature. It is common for many companies and institutions shut down the air conditioning at night to save money, which could result in shifting peak retention times due to dramatic changes in ambient temperature. Many HPLCs provide the option to control column compartment temperature. If your HPLC does not have this capability a heated column jacket can be purchased from many suppliers. The most common running temperature is 40°C, this places the column compartment well above even the most extreme ambient temperature fluctuations. In addition to maintaining constant temperature, temperature can be used to influence the chromatographic separation. No chromatographic study is complete without a temperature study. In our experience higher temperature is better, peaks will be sharper and elute earlier. It is not too uncommon to perform chromatography at 60°C and some daredevils even go to 80°C. Remember though that higher temperature will lead to a shorter column lifetime and some columns may not be able to tolerate 60°C. Consult the manufactures recommendations when experimenting with high temperature. After your runs are complete for the day it is advised that you turn off your column heater since high temperature leads to stationary phase deterioration.
Preparing for the First Run of the Day One observation is that if you start up a reverse phase analysis from a dead stop with a column that has perhaps been sitting in high aqueous conditions for up to 10 hours the analysis will give irreproducible results. Conventional wisdom has it, you want to first flush the column with the highest % organic of your method for at least 3 column volumes and then bring it back to the equilibrating condition. This practice may have the advantage of getting you to standard equilibration conditions faster and it will also clean your column. A better alternative is to make the first run a blank run (or "preparation run") and then the next run can be your real analysis. We prefer the second option because it should get you to the standard starting conditions more accurately. However, often, if we are in a hurry and the first option is quicker, well.....
We store our columns in 50/50 methanol/water without any acid. If you are using a salt, unlikely in LC/MS, wash your entire system, solvent bottles, HPLC, solvent lines, and column, into a non-salt containing solvent. Salt may precipitate out and plug your HPLC or column or may cause corrosion. Usually we flush with pure water first then leave the system in 50/50 methanol: water. Some salts may precipitate out in high organic so an initial water wash is advised. The 50/50 methanol:water solution helps to stop bacterial growth which can muck up your system. Take care of your HPLC, it's the right thing to do!
How Much Protein Should I Load?
|
| Links to Related IonSource Material |
| Calculating
the Appropriate Flow Rates for Columns of Differing Diameters, Maintaining
Linear Velocity
HPLC
Tubing Volume Calculator |
| Links
to External HPLC Information
Vydac's, "The Handbook of Analysis and Purification of Peptides and Proteins by Reversed-Phase HPLC" Quality HPLC Links from LCResources |
|
|
| Important
Safety Information: Triflouroacetic acid, formic acid,
heptaflouobutyric acid and acetic acid are all very caustic reagents.
Acetonitrile, methanol, and propanol are harmful solvents Consult the material safety data sheets
(MSDS) that come with these reagents and get
the permission of the safety officer at you company or institution
before performing these experiments. Always wear the appropriate safety
apparel; safety glasses, lab coat, and gloves. Use a fume hood when
appropriate. If you are not trained in laboratory safety you should
not attempt these procedures. Read our disclaimer, follow the
link at the bottom of this page.
|
|
home | disclaimer visitors |