|
Small Molecule Drug Metabolism
|
IV. Are there common motifs (consensus sites) on molecules where metabolism occurs? |
|
As is pointed out, small molecule drugs are usually lipophilic substances that can penetrate cell membranes to reach the site of action, and drug metabolism is a process of introducing hydrophilic functional groups onto the drug molecule. The most common phase I reactions are oxidative processes that involve cytochrome P450 enzymes. These enzymes are a super family of proteins found in all living organisms. These enzymes catalyze the following reactions: aromatic hydroxylation; aliphatic hydroxylation; N-, O-, and S-dealkylation; N-hydroxylation; N-oxidation; sulfoxidation; deamination; and dehalogenation. These enzymes are also involved in a number of reductive reactions, generally under oxygen-deficiency condition. Hydrolysis is also observed for a wide variety of drugs. The enzymes involved in hydrolysis are esterases, amidases, and proteases. These reactions generate hydroxyl or amine groups, which are suitable for phase II conjugation. Phase II conjugation introduces hydrophilic functionalities such as glucuronic acid, sulfate, glycine, or acetyl group onto the drug or drug metabolite molecules. These reactions are catalyzed by a group of enzymes called transferases. Most trasferases are located in cytosol, except the one facilitates glucuronidation, which is a microsomal enzyme. This enzyme, called uridine diphosphate glucuronosyltransferase (UGTs), catalyzes the most important phase II reaction: glucuronidation. Glucuronic acid contains a number of hydroxyl groups and one carboxylic acid functionality. This molecule is extremely hydrophilic, and improves the hydrophilicity of a drug molecule when they are covalently bound. The following is a partial list of common metabolism motifs :
|
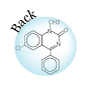 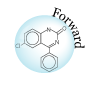
|
|
|
|
IonSource
Home Page | terms of use
(disclaimer) |