|
|
||||
|
Interpreting
Electrospray Mass Spectra |
||||
| - | ||||
| - | ||||
| Electrospray Ion Generation | ||||
|
|
||||
|
|
 |
|||
|
|
- | |||
| Electrospray | Example | |||
|
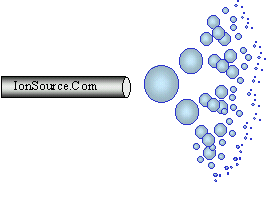
Droplets are generated when a high voltage is
applied to a liquid stream, this technique is known as electrospray.
For example the liquid stream could be the effluent from an HPLC. This method for droplet generation has been used in various applications
since the early part of the 1900s. In electrospray
larger droplets explode into smaller droplets and so on until the
analyte enters the gas phase as an ion. Pure electrospray in the context
of mass spectrometry is accomplished without a nebulizing gas. At
higher LC flow rates a sheath gas is used to
aid in the nebulization process. Some call this method "pneumatically
assisted electrospray."
|
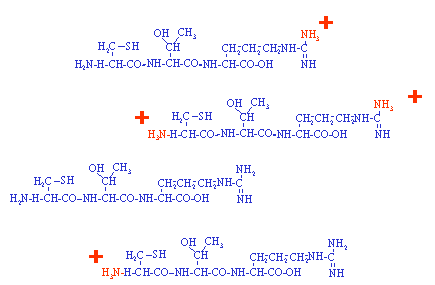
In this example a single peptide is ionized to produce a population of
charged and uncharged peptides. The number of positive charges that
a molecule can support is generally related to the number of basic sites on the
molecule. In positive ion mode the analyte is sprayed at
low pH to encourage positive ion formation. In negative ion the
analysis is normally carried out well above a molecules isoelectric point
to deprotonate the molecule. The basic principle of all mass
spectrometers is that a molecule must be charged (ionized) before the mass spectrometer
can influence it in an electric field. The next page shows how this
population of molecules might appear on a mass spectrum.
Note: Most peptides obtained from a trypsin digest have two potential sites for protonation, the amino terminus and the basic C-terminal residue, lysine or arginine. |
|||
| - | ||||
 |

|
|||
|
- |
||||
| - | ||||
|
- |
||||
|
home
| disclaimer |
||||