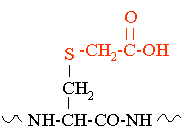|
An IonSource Tutorial |
||
| Monograph 0004 | ||
| S-Carboxymethylation of Cysteine | ||
| Introduction | ||
| . | ||
|
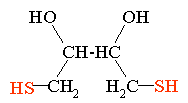
Introduction: Cysteine residues in proteins are reactive and readily form disulfide bonds with other cysteine residues. Reduction and S-carboxymethylation, (RCM), is used to cap cysteine residues and prevent them from reacting and forming disulfide linkages. The RCM reaction is normally used as a prelude to enzymatic digestion of a protein or as a prelude to HPLC or LC/MS analysis of cysteine containing peptides. S-carboxymethylation makes it easier for proteolytic enzymes to more efficiently and completely digest a protein. In Addition the RCM reaction is used to simplify reverse phase peptide maps. A peptide map that contains multiple disulfide linked peptides makes the analysis and identification of those peptides difficult. Another complication is that most enzyme digests are performed at elevated pH. At high pH disulfide bonds can scramble further complicating the peptide mapping analysis by the introduction of the artifctual disulfide linkages. Protein Sequencing and AAA: Unmodified cysteine is destroyed in N-terminal protein sequencing (Edman degradation) and in amino acid analysis procedures. Carboxymethylated cysteine is observed in these analyses, therefore, this and other alkylating procedures are often used prior to protein sequencing and AAA. Useful Note: If the purpose is to obtain a reduced LC/MS mass spectrum of a peptide or protein it is possible to just perform the reduction and shoot this mixture onto a reversed phase column and obtain the desired reduced peptide result. The high pH of the injection mixture will not significantly harm the silica of the reverse phase column because the residence time of the high pH buffer is extremely short. |
|
|
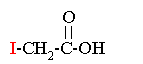
Iodoacetic Acid |
||
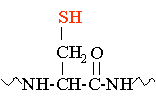 Cysteine |
||
|
|
||
|
. |
||
|
TOC Introduction Reaction Method Method Notes Why Reduce? RCM Compound Data Base Order Reagents References |
||
| - | ||
| - | ||
|
home
| disclaimer |
||